Alzheimer’s disease: the identification of 75 genetic risk factors brings new insights
Researchers in Europe, the US and Australia have identified 75 regions of the genome that are associated with Alzheimer's disease. Forty-two of these regions are novel, meaning that they have never before been implicated in the disease.
April 4th 2022
Identifying genetic risk factors for Alzheimer's disease is essential if we are to improve our understanding and treatment of it. Progress in human genome analysis along with genome-wide association studies1 are now leading to major advances in the field. Researchers in Europe, the US and Australia have identified 75 regions of the genome that are associated with Alzheimer's disease. Forty-two of these regions are novel, meaning that they have never before been implicated in the disease. The findings, published in Nature Genetics, bring new knowledge of the biological mechanisms at play and open up new avenues for treatment and diagnosis.
Alzheimer's disease is the most common form of dementia, affecting around 8 million people in Europe. This complex, multifactorial disease, which usually develops after the age of 65, has a strong genetic component. The majority of cases are thought to be caused by the interaction of different genetic predisposition factors with environmental factors.
Although our understanding of the disease continues to improve, there is no cure at this time. The medications available are mainly aimed at slowing cognitive decline and reducing certain behavioral disorders. In order to better understand the origins of the disease, one of the major challenges of research is to better characterize its genetic risk factors by identifying the pathophysiological processes at play2, and thereby propose novel therapeutic targets.
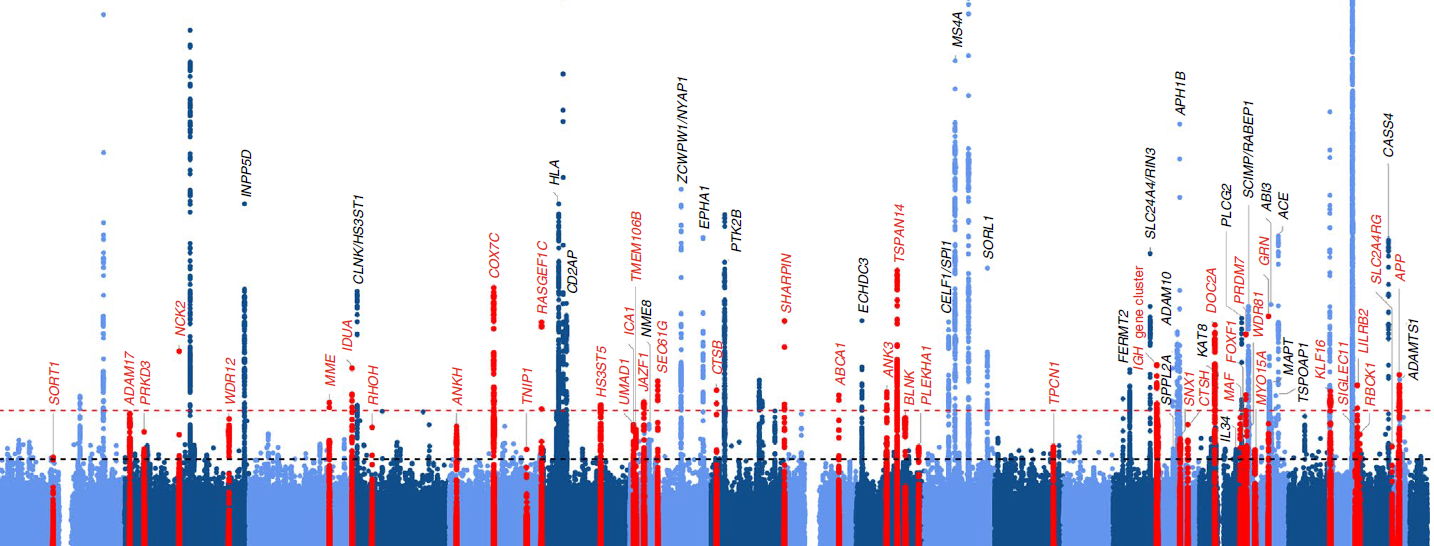
As part of an international collaboration, researchers from the VIB-UAntwerp Center for Molecular Neurology conducted a genome-wide association study (GWAS) on the largest Alzheimer’s patient group set up until now3.
Encouraged by advances in genome analysis, these studies consist of analyzing the entire genome of tens of thousands or hundreds of thousands of individuals, whether healthy or sick, with the aim of identifying genetic risk factors associated with specific aspects of the disease.
Using this method, the scientists were able to identify 75 regions (loci) of the genome associated with Alzheimer's, 42 of which had never previously been implicated in the disease.
"Following this major discovery, we characterized these regions in order to give them meaning in relation to our clinical and biological knowledge, and thereby gain a better understanding of the cellular mechanisms and pathological processes at play," explains prof. Kristel Sleegers (VIB-UAntwerp).

Highlighting pathological phenomena
In Alzheimer's disease, two pathological brain phenomena are already well documented: namely, the accumulation of amyloid-beta peptides and the modification of the protein Tau, both of which aggregate in affected brain tissue.
Here, the scientists confirmed the importance of these pathological processes. Their analyses of the various genome regions confirm that some are implicated in amyloid peptide production and Tau protein function.
Furthermore, they also reveal that dysfunction of innate immunity and of microglia (immune cells present in the central nervous system that play a "trash collector" role by eliminating toxic substances) is at play in Alzheimer's disease.
Finally, this study shows for the first time that the tumor necrosis factor alpha (TNF-alpha)-dependent signaling pathway is involved in disease4.
These findings confirm and add to our knowledge of the pathological processes involved in the disease and open up new avenues for therapeutic research. For example, they confirm the utility of the following: the conduct of clinical trials of therapies targeting the amyloid precursor protein, the continuation of microglial cell research that was initiated a few years ago, and the targeting of the TNF-alpha signaling pathway.
Risk score
Based on their findings, the researchers devised a genetic risk score in order to better evaluate which patients with cognitive impairment will, within three years of its clinical manifestation, go on to develop Alzheimer's disease.
"While this tool is not at all intended for use in clinical practice at present, it could be very useful when setting up therapeutic trials in order to categorize participants according to their genetic risk and improve the evaluation of the medications being tested," explains Sleegers.
In order to validate and expand their findings, the team would now like to continue its research in an even broader group. Beyond this exhaustive characterization of the genetic factors of Alzheimer's disease, the team is also developing numerous cellular and molecular biology approaches to determine their roles in its development.
Furthermore, with the genetic research having been conducted primarily on Caucasian populations, one of the considerations for the future will be to carry out the same type of studies in other ethnic groups in order to determine whether the risk factors are the same from one population to the next, which would reinforce their importance in the pathophysiological process.
1 These studies consist of analyzing the entire genome of thousands or tens of thousands of people, whether healthy or sick, to identify genetic risk factors associated with specific aspects of the disease.
2 All functional problems caused by a particular disease or condition.
3 Here, the researchers were interested in the genetic data of 111,326 people who were diagnosed with Alzheimer's disease or had close relatives with the condition, and 677,663 healthy "controls". These data are derived from several large European cohorts grouped within the European Alzheimer & Dementia BioBank (EADB) consortium.
4 Tumor necrosis factor alpha is a cytokine: an immune system protein implicated in the inflammation cascade, particularly in tissue lesion mechanisms.
Publication
New insights into the genetic etiology of Alzheimer’s disease and related Dementias
Bellenguez et al. Nature Genetics 2022