How garbage disposal clogs in cells can lead to Alzheimer’s
Leuven, 25 May 2023 - Although millions of people are affected by Alzheimer’s disease, there are still many unknowns about the underlying mechanisms of the disorder. In their latest research, scientists from the lab of Wim Annaert (VIB-KU Leuven) uncovered a piece of the mechanistic puzzle by showing how a gene variation in the neurons’ ‘garbage disposal’ system can lead to Alzheimer’s disease.
In Alzheimer’s disease, a protein called amyloid precursor protein is incorrectly processed, resulting in the formation of toxic amyloid-β peptides. These peptides can build up in the brain and form plaques, which are a hallmark of Alzheimer’s disease. The accumulation of these toxic peptides in neurons happens early in the disease, even before symptoms appear. This may originate from the fact that neurons gradually lose the capacity to degrade this cellular ‘garbage’.
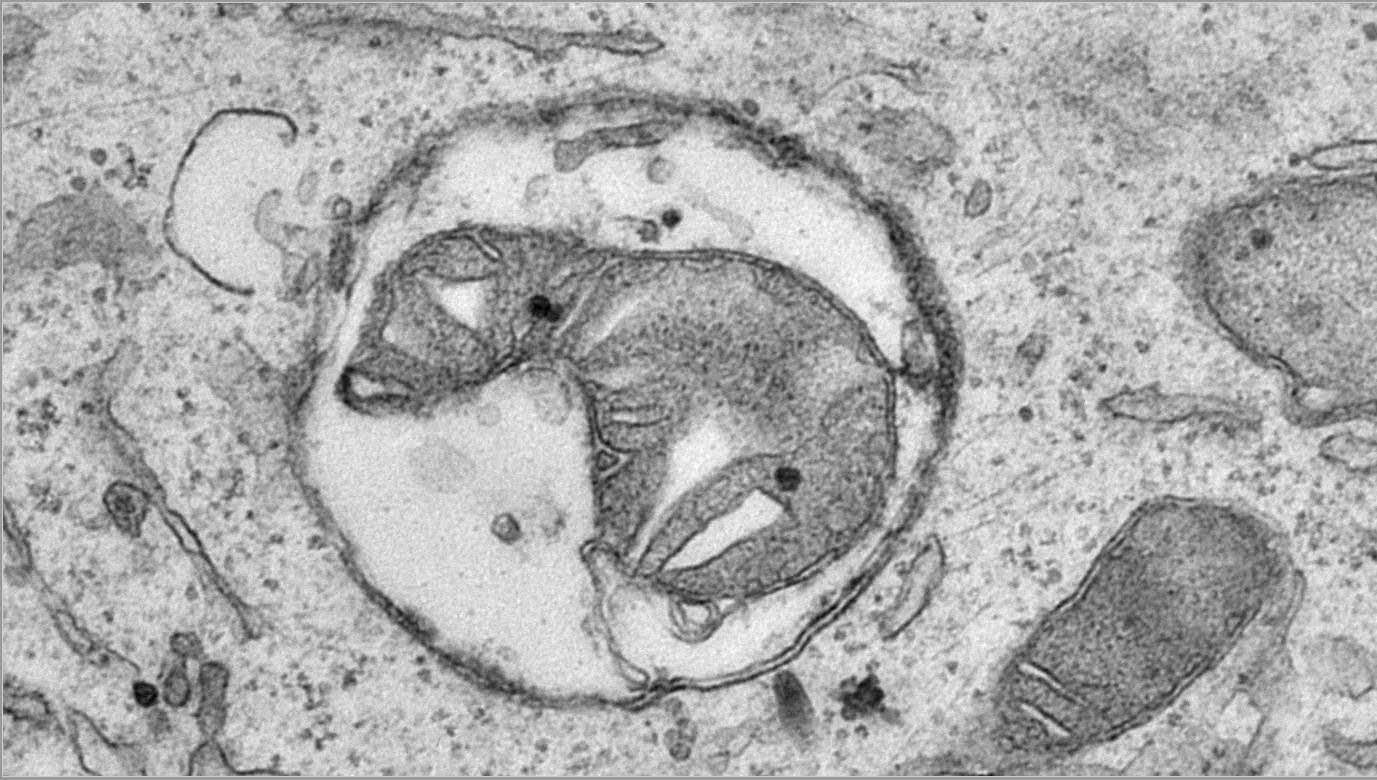
The lysosomal system plays a crucial role in breaking down and recycling old or damaged materials in cells, including neurons. It helps to keep the neurons clean and prevents the buildup of harmful waste that can damage and kill them. Healthy lysosomes are essential for the survival of neurons throughout a person’s life.
When people age, it is precisely this lysosomal system that starts to become vulnerable, a process that is enhanced in the early stages of neurodegenerative diseases like Alzheimer’s disease. Several risk genes related to the lysosomal system have also been linked to Alzheimer’s disease. But we still don't fully understand how these genes cause lysosomes to fail.
One such gene, called phospholipase D3 (PLD3), has been studied by Wim Annaert and his team. Patients with Alzheimer's have lower levels of this protein, and a small percentage even carry certain variants of the PLD3 gene that increase the risk of developing the disease.
“For most risk genes in Alzheimer’s disease, we lack profound knowledge on how the involved proteins actually function in the neuron. However, this is critical to understand how disease onsets and progresses. For one of them, PLD3, we now discovered the mechanism of how its failure in lysosomes is connected to pathological hallmarks of Alzheimer’s disease.” - Wim Annaert (VIB-KU Leuven)

A STING in mitochondrial DNA breakdown
In the brain, PLD3 is highly present in the lysosomes of neurons, where it helps to break down DNA fragments. However, why these DNA fragments were present in lysosomes and why failing to degrade DNA would trigger Alzheimer’s pathology was not yet known.
Zoë Van Acker, first author of the study: “It is exciting to now have answers to these questions, starting with identifying that most of the DNA actually comes from mitochondria, or the neuron’s powerhouses. Mitochondria have a rapid turn-over in the energy-consuming neurons, and ‘used up’ mitochondria need to be recycled through lysosomes and replaced by young ones. However, when PLD3 is defective, the DNA from mitochondria fails to become degraded in lysosomes and accumulates. This clearly triggers a chain of events that results in a clogging of the lysosomal system.”
The research team found that the excess mitochondrial DNA leaks out of lysosomes, similar to an oil leak. This leak causes neurons to activate certain signaling pathways, such as the cGAS-STING pathway. Unfortunately, instead of stopping this leak, STING activation unintentionally makes the problem worse. It adds more waste and cell components to the overwhelmed lysosomes, which cannot handle the increased load. As a result, the lysosomes fail to break down their usual waste, including toxic substances like amyloid peptides and other toxic fragments of the amyloid precursor protein, which are known to harm neurons and contribute to the development of amyloid plaques associated with Alzheimer’s disease. This accumulation of toxic substances further damages the neurons and may therefore contribute to the worsening of the pathology.
Overall, this research sheds new light on the complex interactions between different neuronal processes that can lead to Alzheimer’s disease. By understanding these interactions, scientists may be able to develop new treatments to slow or even prevent the development of the disease.

Publication
Phospholipase D3 degrades mitochondrial DNA to regulate nucleotide signaling and APP metabolism. Van Acker, et al. Nature Communications, 2023. https://doi.org/10.1038/s41467-023-38501-w
India Jane Wise
Joran Lauwers
About the VIB-KU Leuven Center for Brain & Disease Research
Scientists at the VIB-KU Leuven Center for Brain & Disease study how brain cells are organized and how they communicate with each other. These mechanisms reveal and provide insights into what goes wrong in brain diseases such as Alzheimer's, Parkinson's, ALS, and dystonia. This basic work should ultimately lead to new drugs for use against these currently incurable diseases.