New mechanism uncovered in early stages of Alzheimer's disease
APP-CTFs disrupt organellar communication, interfering with cellular homeostasis
Leuven, 16 April 2024 – Alzheimer's disease (AD) remains one of the most challenging and prevalent neurodegenerative disorders, affecting millions of individuals worldwide. In a new study published in Developmental Cell, researchers from the lab of Wim Annaert (VIB-KU Leuven) have identified a novel mechanism potentially connected to the early stages of AD. They demonstrated that a fragment of the amyloid precursor protein (APP), called APP-CTF, disrupts communication between cellular compartments crucial for calcium storage and waste disposal, which could be an early event preceding neuronal cell death. These findings, with potential implications for the development of new AD treatments, suggest that preventing APP-CTF accumulation needs to be taken into account to develop more effective treatments.
Alzheimer's disease is characterized by the progressive loss of cognitive function, memory impairment, and behavioral changes. One of the visible features in the brains of people with Alzheimer's disease is the formation of amyloid plaques – clumps of β-amyloid (Aβ) peptides, which are degraded products of amyloid precursor protein (APP). These Aβ-fragments accumulate in neurons early in the disease, even before cognitive decline is observed.
New research, however, suggests that there might even be earlier events happening in the AD brain before plaque formation and that the APP protein plays a role in these early stages. The mechanism behind this remained a mystery until now.
In their latest study, the lab of Wim Annaert at the VIB-KU Leuven Center for Brain & Disease Research identified a mechanism explaining how APP may contribute to these early stages of AD. This discovery could lead to a new direction in AD research and treatment approaches.
Disrupting cellular communication
APP is found in the cell membranes of brain cells. The brain constantly produces new APP molecules while breaking down and removing old ones. This process involves enzymatic scissors, with gamma-secretase being the final one that generates the well-known and well-studied Aβ peptides in AD.
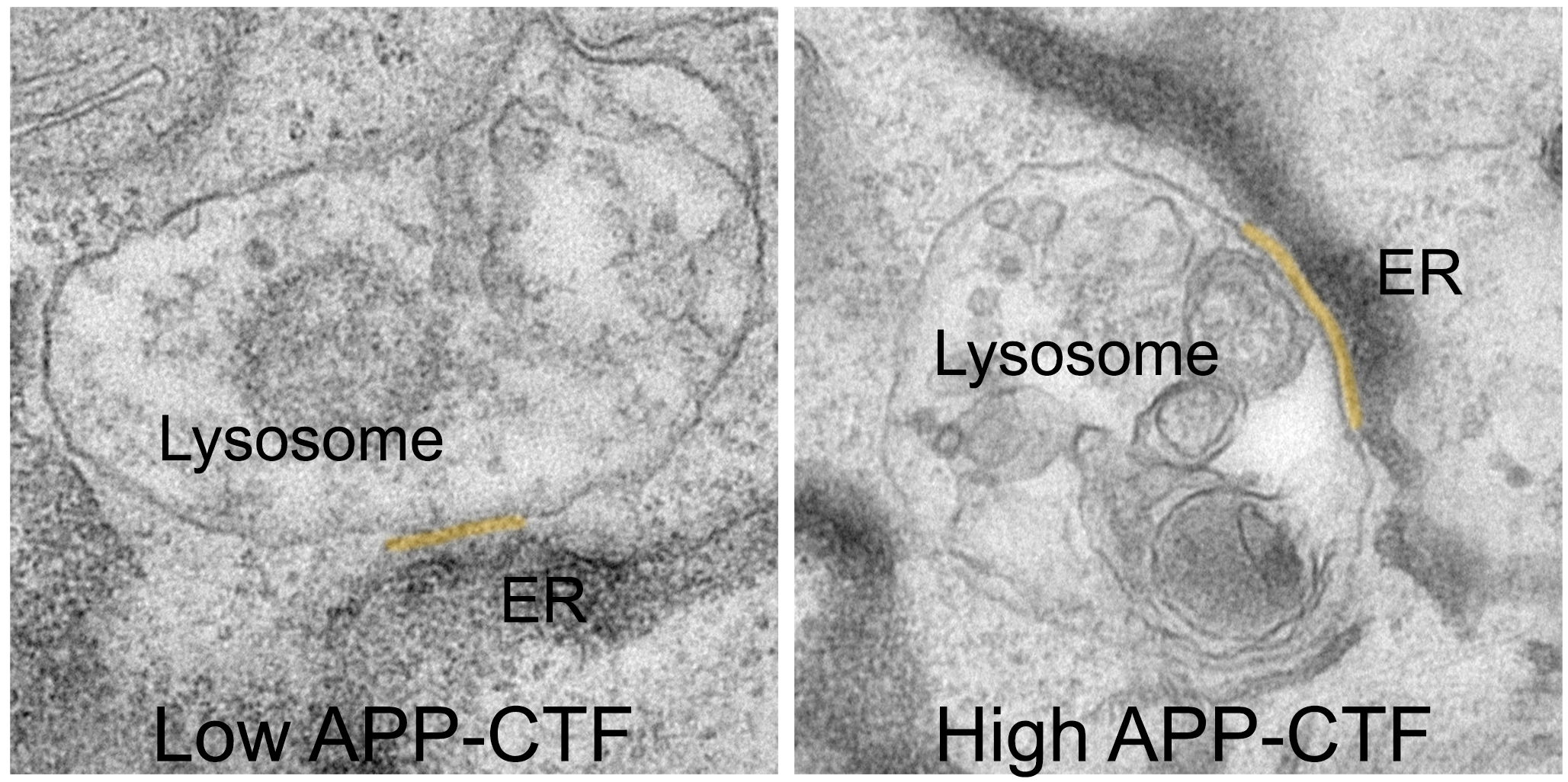
For a long time, it was thought that blocking gamma-secretase would be the logical step to prevent the production of toxic Aβ fragments. However, this leads to the accumulation of their precursor, the APP-C-Terminal Fragments, or APP-CTFs. Now, the researchers have discovered that these fragments are also toxic to neurons. They appear to accumulate between the endoplasmic reticulum (ER), the compartment that is crucial for lipid synthesis and calcium storage, and the lysosomes, the so-called ‘waste bins’ of neurons, which are critical for degrading the cell’s waste products.
“By doing so, APP-CTFs disrupt the delicate balance of calcium within lysosomes," explains Dr. Marine Bretou, first author of the study. "This disruption triggers a cascade of events. The ER can no longer effectively refill lysosomes with calcium, leading to a buildup of cholesterol and a decline in their ability to break down cellular waste. This results in the collapse of the entire endolysosomal system, a crucial pathway for maintaining healthy neurons."
The new study further supports that the APP-CTFs resulting from suppressing gamma-secretase might actually be the culprit behind endolysosomal dysfunction, as observed in the very early stages of AD.
A paradigm shift in understanding the early stages of AD pathogenesis
This research significantly advances our comprehension of the potential causes of disease in the early stages of AD. A remarkable outcome of this study is that these early stages could be caused by another fragment of the same APP molecule rather than Aβ. This has significant implications for the current therapeutic approaches that aim to clear the AD brain from amyloid plaques, as they tend to ignore the toxic effects of other fragments. Other attempts focus on tau proteins or neuroinflammation, which are other hallmarks of AD progression that target later events. However, early intervention is likely the key to stopping or even preventing AD.
"The failure of clinical trials using gamma-secretase inhibitors may be explained by the fact that we were focusing on only one culprit and at a too late stage in the disease," explains Prof. Wim Annaert, lead author of the study. “Our research findings suggest that gamma-secretase modulators, which can help promote clearance of toxic APP-CTFs without blocking the enzyme completely, may be a more relevant target for early intervention in AD. The key might be finding the right balance between APP-CTF clearance and plaque prevention.”
Looking ahead, the scientists are joining efforts with colleagues to develop these modulators based on these novel insights and will continue exploring cellular homeostasis in the early stages of AD.
Publication
Accumulation of APP C-terminal fragments causes endolysosomal dysfunction through the dysregulation of late endosome to lysosome-endoplasmic reticulum contact sites. Bretou, et al. Developmental Cell, 2024. DOI: 10.1016/j.devcel.2024.03.030
The research was funded by VIB, KU Leuven, Fondation Recherche Alzheimer - Stichting Alzheimer Onderzoek (STOPALZHEIMER.BE), the Alzheimer’s Association, and the FWO Program.
India Jane Wise
Joran Lauwers
About the VIB-KU Leuven Center for Brain & Disease Research
Scientists at the VIB-KU Leuven Center for Brain & Disease study how brain cells are organized and how they communicate with each other. These mechanisms reveal and provide insights into what goes wrong in brain diseases such as Alzheimer's, Parkinson's, ALS, and dystonia. This basic work should ultimately lead to new drugs for use against these currently incurable diseases.
About KU Leuven
KU Leuven is Europe’s most innovative university (Reuters) and ranks 45th in the Times Higher Education World University Rankings. As Belgium's largest university, KU Leuven welcomes 60,000 students from over 140 countries. Its 7,000 researchers are active in a comprehensive range of disciplines. KU Leuven is a founding member of the League of European Research Universities (LERU) and has a strong European and international orientation. University Hospitals Leuven, its network of research hospitals, provides high-quality healthcare and develops new therapeutic and diagnostic insights with an emphasis on translational research.