.png)
New study discovers how neurons die in Alzheimer’s disease
Neurons are triggered into programmed cell death following exposure to amyloid plaques and tau tangles
Leuven, 15 September 2023 – A research team led by Prof. Bart De Strooper (VIB-KU Leuven and the UK Dementia Research Institute) and Dr. Sriram Balusu (VIB-KU Leuven) has finally discovered how neurons die in Alzheimer’s disease. Subject of scientific discussion for the past decades, a breakthrough research paper published in Science illustrates how neurons initiate a programmed form of cell death, known as necroptosis, when they are exposed to amyloid plaques and tau tangles – the hallmark misfolded proteins implicated in Alzheimer’s. More importantly, the research team was able to prevent the death of neurons, rescuing them in the process. The discovery opens new pathways for potential future treatments.
Alzheimer’s disease (AD) is one of the most common forms of dementia, accounting for 60 to 70% of dementia diagnoses. Each year, between six and seven million patients are diagnosed with AD. As a debilitating disease that often comes with a major emotional and psychological burden for both patients and their families, it represents a growing societal challenge and has been classified as public health priority by the World Health Organisation (WHO). Even though the past few years have seen some developments in treatments that slow down disease progression, there currently is no cure for AD, as the underlying cause of the disease is still not fully understood.
A recent breakthrough publication published in Science and coordinated by a research team led by Prof. Bart De Strooper and Dr. Sriram Balusu now lifts part of the veil over the biological mechanisms underpinning this debilitating disease.
Prof. Bart De Strooper, Group Leader at the VIB-KU Leuven Center for Brain and Disease Research and the UK Dementia Research Institute at University College London: “Our study sheds light on the previously murky waters of Alzheimer’s disease, revealing a potential key player in neuronal loss – an RNA gene called MEG3, and the process of necroptosis. These findings are an important step forward in furthering our understanding of the basic mechanisms underlying this complex and often misunderstood disease.”
.png)
A new model to crack the Alzheimer’s enigma
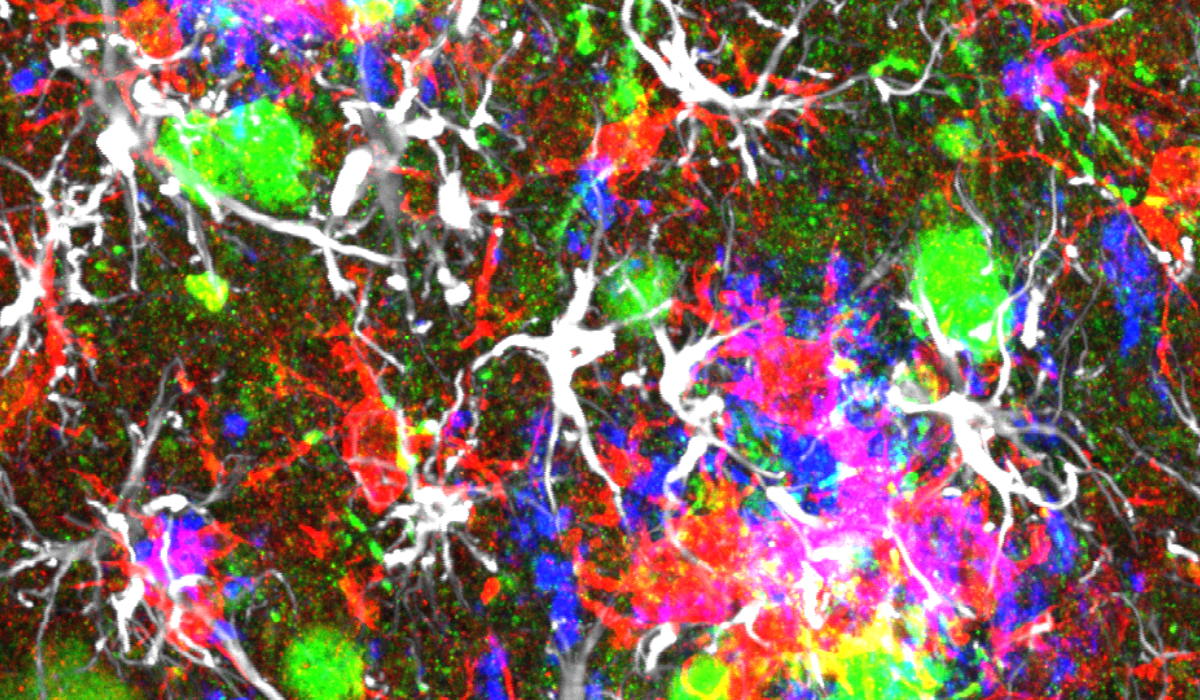
One of the key challenges in understanding Alzheimer’s disease (AD) has been connecting its defining hallmarks - amyloid plaques, tau tangles, and death of neurons - to each other. Most mouse models used in research couldn’t naturally replicate these features, leaving scientists with unanswered questions about how they relate to disease progression.
“To bridge this gap, we created a new model,” says Dr. Sriram Balusu, postdoctoral researcher at the De Strooper lab and first author of the paper. “We implanted both healthy human and mouse neurons into the brains of AD mouse models. The human cells degenerated much like their counterparts in the human brain, allowing us to study them during brain aging and shine a new light on the processes underlying AD.”
Remarkably, only the human neurons, and not their rodent counterparts, displayed AD features seen in the brains of patients, including tau tangles, and significant neuronal cell loss. This suggests that there may be human-specific factors at play in AD that standard mouse models can’t replicate. Understanding why mouse neurons are more resilient to amyloid pathology will not only help model the disease better but might also stimulate research into pathways that protect against neurodegeneration.
The culprit behind brain cell loss
Using their new model, the team probed deeper, seeking answers on how neurons die in AD. The study revealed a critical breakthrough: a pathway known as necroptosis, a form of programmed cell death, was activated in the model, leading to the death of neurons.
But the discovery went even further. The researchers saw that levels of a molecule known as MEG3 were strongly increased in human neurons, as seen in AD patients. Strikingly, just the presence of MEG3 alone was enough to trigger the pathway of necroptosis in human neurons in a lab setting. The study also found that by reducing MEG3 and preventing necroptosis, researchers could, in turn, prevent the death of cells. More research is needed to understand how exactly MEG3 triggers necroptosis, but this discovery represents a crucial advancement in understanding how Alzheimer’s leads to the loss of neurons in the brain.
“Necroptosis is already an active area of drug development in cancer and ALS,” says Bart De Strooper, who has been studying Alzheimer’s at the VIB-KU Leuven Center for Brain & Disease research for over 30 years, in addition to acting as Director of the UK Dementia Research Institute until this year. “While there’s much more to explore, our findings open up promising avenues for potential therapies targeting AD, alongside traditional approaches aimed at amyloid and tau.”
Publication
MEG3 activates necroptosis in human neuron xenografts modeling Alzheimer's disease. Balusu et al. Science, 2023. DOI: 10.1126/science.abp9556
India Jane Wise
Molly Andrews
Questions from patients
A breakthrough in research is not the same as a breakthrough in medicine. The realizations of VIB researchers can form the basis of new therapies, but the development path still takes years. This can raise a lot of questions. That is why we ask you to please refer questions in your report or article to the email address that VIB makes available for this purpose: patienteninfo@vib.be. Everyone can submit questions concerning this and other medically-oriented research directly to VIB via this address.
About the VIB-KU Leuven Center for Brain & Disease Research
Scientists at the VIB-KU Leuven Center for Brain & Disease study how brain cells are organized and how they communicate with each other. These mechanisms reveal and provide insights into what goes wrong in brain diseases such as Alzheimer's, Parkinson's, ALS, and dystonia. This basic work should ultimately lead to new drugs for use against these currently incurable diseases.
About the UK Dementia Research Institute
The national UK Dementia Research Institute (UK DRI) is the single biggest investment in dementia research in the UK. Established in 2017 by its founding funders, the Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK, the multi-million-pound Institute is hosted across six leading UK universities: University of Cambridge, Cardiff University, University of Edinburgh, Imperial College London and King’s College London, with its central hub at UCL. The UK DRI works on ways to prevent, treat and care for people with all types of dementia, and ways to keep the brain healthy. www.ukdri.ac.uk


