Scientists create protein ‘seeds’ that trigger key pathological features of ALS and frontotemporal dementia
28 March 2024, Leuven, Belgium — Accumulation of a protein called TDP-43 is a key feature of ALS and frontotemporal dementia. In a newly published study, researchers report ‘seeding’ this accumulation through fragments of the culprit protein created in the lab. The findings provide further evidence for a prion-like paradigm wherein protein aggregation occurs in a templated fashion. This breakthrough provides the research field with a powerful way to model and study the mechanisms driving neurodegeneration.
TAR DNA-binding protein 43, better known as TDP-43, is a protein found in almost all human cells, where it plays essential roles in regulating gene expression, RNA processing, and cellular stress responses. Under normal conditions, TDP-43 helps maintain the health and function of neurons by controlling which genes are turned on or off and how their messages are translated into proteins.
However, TDP-43 is infamous for its role in several neurodegenerative diseases, including ALS and frontotemporal dementia, but also Alzheimer’s disease.
From the nucleus to the cytoplasm
“TDP-43 pathology is considered a defining feature in nearly all ALS cases and about half of frontotemporal dementia cases,” explains professor Sandrine Da Cruz, group leader at the VIB-KU Leuven Center for Brain & Disease Research. “In the brains of these patients, TDP-43 somehow mislocalizes, accumulates in the cytoplasm where it forms insoluble inclusions, and is depleted from the nucleus.”
Despite its critical role, the exact processes driving TDP-43 dysfunction remain poorly understood—an urgent gap that researchers like Da Cruz are actively working to fill. The havoc and widespread neuronal death that ensues following TDP-43 mislocalization is most likely due to a combination of both disruption of TDP-43’s normal activities within the nucleus as well as the toxic effect of the cytoplasmic inclusions.
“Part of the reason why the underlying mechanisms are still poorly understood is that we lack model systems that reliably recapitulate both the nuclear depletion of TDP-43 and its cytoplasmic aggregation,” says Da Cruz.
Seeds for aggregation
Building on recent reports that autopsied brain material from patients with TDP-43 inclusions can induce accumulation of insoluble TDP-43 in cells and transgenic mouse models, Da Cruz and her team sought a way to reproduce this so-called aggregation ‘seeding’ in the lab.
In a study recently published in Neuron, Da Cruz’s team describes how they produced amyloid-like fibrils from a fragment of TDP-43 and that these fibrils trigger TDP-43 pathology in human cells, including iPSC-derived neurons.
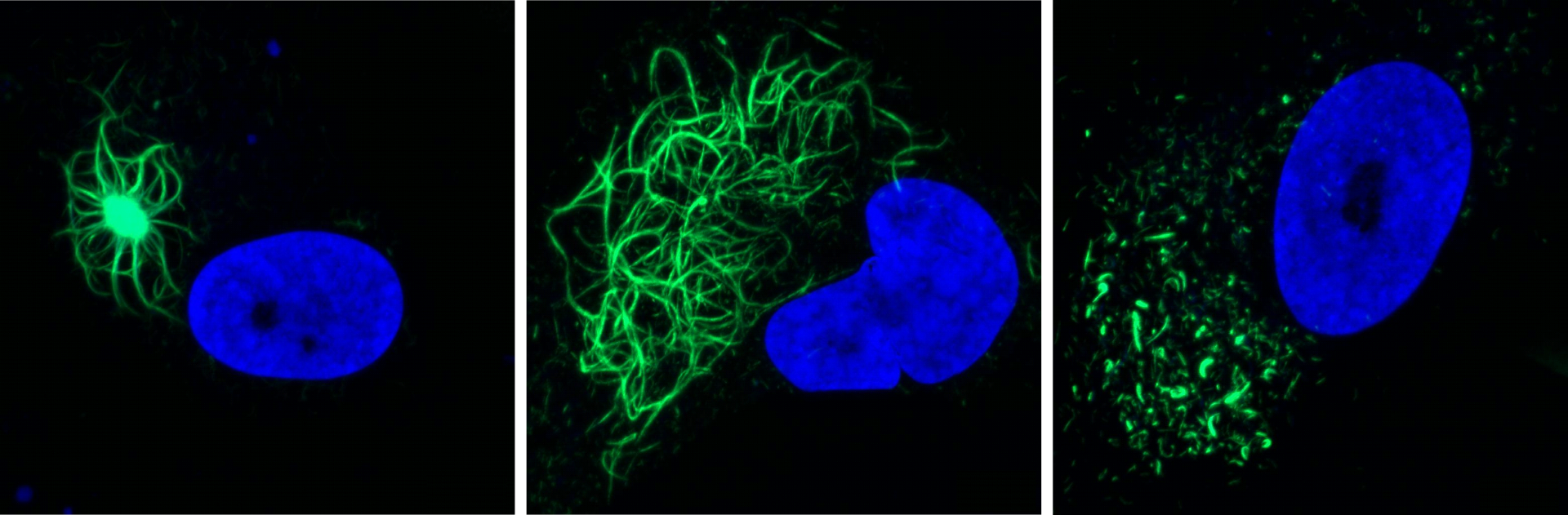
The fibril-induced inclusions recapitulate many of the key hallmarks seen in patients, explains PhD student Jens Rummens: “The TDP-43 aggregates induced by the fibrils exhibited many of the modifications we also see in patient brains, including phosphorylation and ubiquitination. Strikingly, the aggregates were able to recruit endogenous TDP-43 from the nucleus to the cytoplasm.”
The similarities extended to other downstream effects, as the team identified signature gene activity patterns that were previously linked to both aggregation and nuclear loss of TDP-43. The aggregates themselves exhibited the same heterogeneity in morphology as typically seen in patients over time.

Tool for research
The new results strongly suggest that pathology in TDP-43 proteinopathies propagates in a self-templating and prion-like fashion, but many questions remain unanswered. How is TDP-43 ‘trapped in the aggregates? What do they consist of and how do they trigger toxicity? Which additional ‘hits’ are required? What are the effects of TDP-43 mutations? Of age?
So perhaps most importantly, the new study provides scientists with the actual tools to study the different triggers and complex interplay in a controlled system.
Da Cruz: “We have developed a valuable model that displays both aspects of TDP-43 pathology—cytoplasmic aggregation and nuclear depletion. This will be a powerful asset to help researchers across the globe to further unravel TDP-43 induced disease mechanisms and enable us to screen potential drug candidates that modify disease progression.”

TDP-43 seeding induces cytoplasmic aggregation heterogeneity and nuclear loss-of-function of TDP-43
Rummens et al. Neuron 2025
This research was a collaboration between different labs at VIB, KU Leuven and UHasselt, enabled by additional financial support from FWO, the Muscular Dystrophy Association and Stichting Alzheimer Onderzoek - Fondation Recherche Alzheimer (STOPALZHEIMER.BE).


