Researchers develop model that predicts onset of Alzheimer’s disease
Leuven, 5 May 2025 – A group of researchers in the lab of Prof. Lucía Chávez Gutiérrez (VIB-KU Leuven) have unraveled the genetic contributions to familial Alzheimer’s Disease development and revealed how specific mutations act as a clock to predict the disease age of onset. These insights, published in Molecular Neurodegeneration, could aid clinicians to improve early diagnosis and tailor treatment strategies.
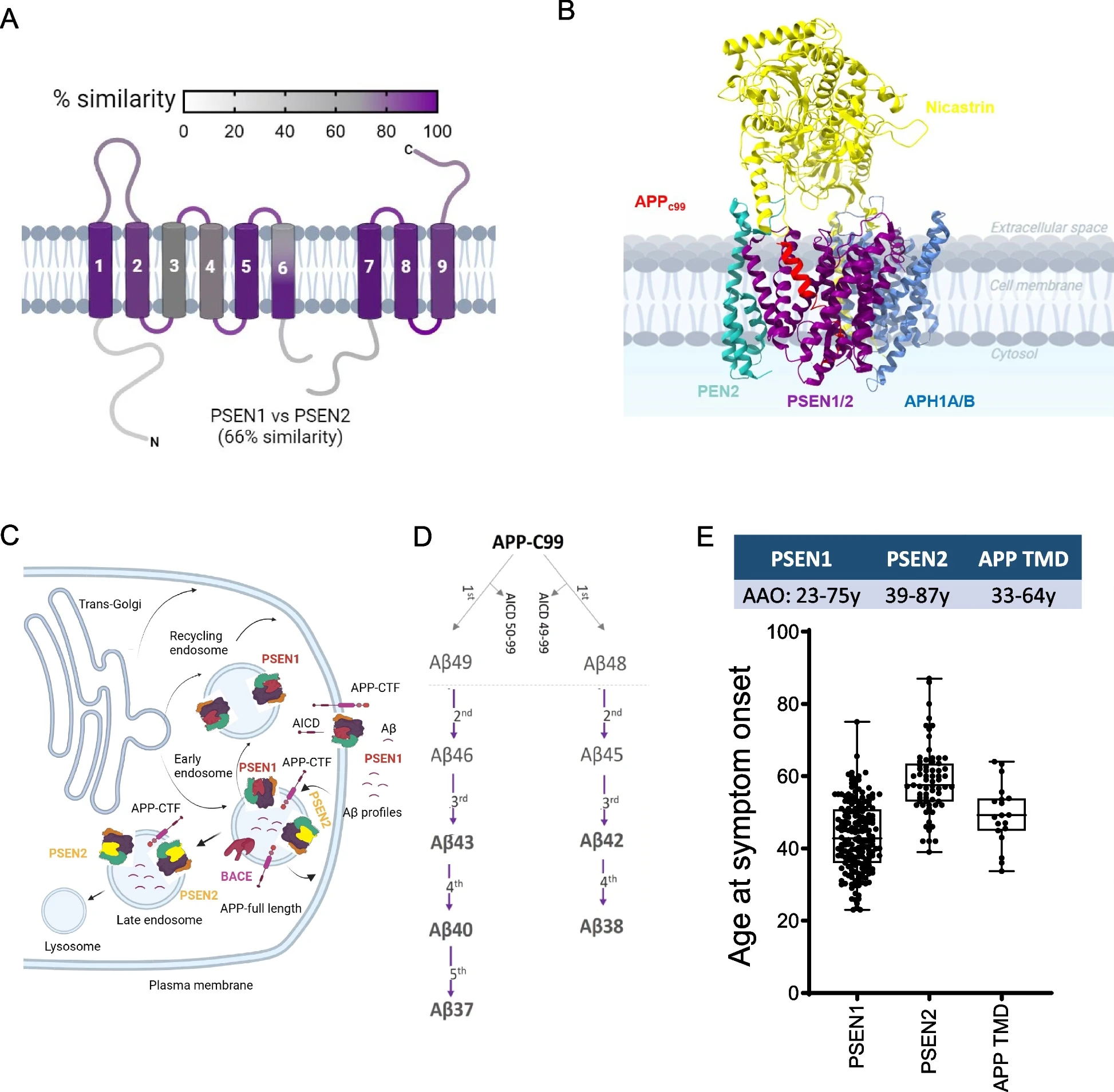
Alzheimer's disease remains one of the most challenging and prevalent neurodegenerative disorders, affecting 50 million people worldwide. To date, the exact cause of the disease is still not fully understood. One of the key visible features in the brains of individuals with Alzheimer's disease is the presence of amyloid plaques. These plaques are formed in the neurons and consist of clumps of misfolded amyloid-β (Aβ, pronounced ‘a-beta’) fragments. These fragments are produced by a sophisticated molecular processing system orchestrated by the γ-secretase enzyme and several key proteins.
Familial Alzheimer’s disease is a rare, early-onset type of Alzheimer’s disease that is caused by mutations in three important genes involved in this system: amyloid precursor protein (APP), Presenilin 1 (PSEN1), or Presenilin 2 (PSEN2). Their exact role in the disease is not well understood and has been debated by scientists for several decades. Understanding more about the link between specific kinds of mutations and the age of onset for familial Alzheimer’s disease could be useful for doctors to make more accurate clinical diagnoses.
"In familial Alzheimer’s disease, patients are often seen to have spontaneous genetic mutations, but until now doctors have not been able to provide patients more specific information about them," explains Prof. Lucía Chávez Gutiérrez. "We have developed a method to experimentally test how likely a mutation is to cause the disease, as well as to predict disease onset."

Mutations acting like ticking clocks
The laboratory of Prof. Lucía Chávez Gutiérrez at the VIB-KU Leuven Center for Brain & Disease Research recently demonstrated that mutations in PSEN1 correlate strongly with age of onset for Alzheimer’s disease. Now, they conducted the same analysis for mutations in all three causal genes: PSEN1, PSEN2, and APP. They found very clear correlations between specific mutations and the age of onset for familial Alzheimer’s disease.
“When we put all of our data together, it gives us a much clearer picture of how each of the causal genes contribute to the development of familial Alzheimer’s disease – we can measure the exact contribution of each gene and even predict when the first symptoms will appear." explains Sara Gutiérrez Fernández, first author of the study.
Alzheimer’s genes: a countdown to onset
For a long time, scientists have understood that the accumulation of longer Aβ peptides in the brain may be involved in triggering the molecular and cellular programs that lead to Alzheimer’s disease. Recent studies,including research from the lab of Prof. Lucía Chávez Gutiérrez, have shown a strong link between the proportion of long-to-short Aβ peptides and Alzheimer’s disease pathogenesis.
In this study, researchers noticed direct and linear relationships between the proportion of long-to-short Aβ fragments and the age of onset of the disease. These parallel relationships shifted across genes, which suggests the presence of a common pathogenic mechanism with gene-specific onset timings.
"Our data predicts that a 12% shift in Aβ profile could delay the age of onset in familial Alzheimer’s disease by up to 5 years," says Prof. Lucía Chávez Gutiérrez. "This highlights the potential of therapies that target γ-secretase in the brain to create shorter forms of Aβ, and in turn delay or prevent disease onset."
From genes to personalized medicine: strategies for early diagnosis and treatment in familial Alzheimer’s
Beyond uncovering key disease mechanisms, the researchers also developed a framework that serves two useful functions for genetic research. First, it can assess how capable a genetic variant is of causing familial Alzheimer’s disease. Second, it can help to identify individuals who carry genetic modifiers or who have been exposed to other environmental factors that influence the expected age of dementia onset.
This dual-role framework enhances the ability of researchers to interpret genetic data and understand the complex interplay of factors influencing familial Alzheimer’s disease progression. Not only that, but it also supports new avenues for therapeutic interventions in familial Alzheimer’s, and potentially in more common forms of the disease.
“We have developed a predictive model for age of onset that could pave the way for personalized approaches to managing familial Alzheimer’s," Sara Gutiérrez Fernández shares. “In the future, this may help clinicians to more effectively design strategies for early diagnosis and treatment for patients with genetic forms of the disease. Our lab is now focused on doing more research with the aim of developing therapies using this model."
Funding
The research (team) at the VIB-KU Leuven Center for Brain & Disease Research was financially supported by the Foundation Flanders (FWO), Foundation Recherche Alzheimer - Stichting Alzheimer Onderzoek (STOPALZHEIMER.BE), VIB, and KU Leuven.
Publication
Joran Lauwers
About the VIB-KU Leuven Center for Brain & Disease Research
Scientists at the VIB-KU Leuven Center for Brain & Disease study how brain cells are organized and how they communicate with each other. These mechanisms reveal and provide insights into what goes wrong in brain diseases such as Alzheimer's, Parkinson's, ALS, and dystonia. This basic work should ultimately lead to new drugs for use against these currently incurable diseases.