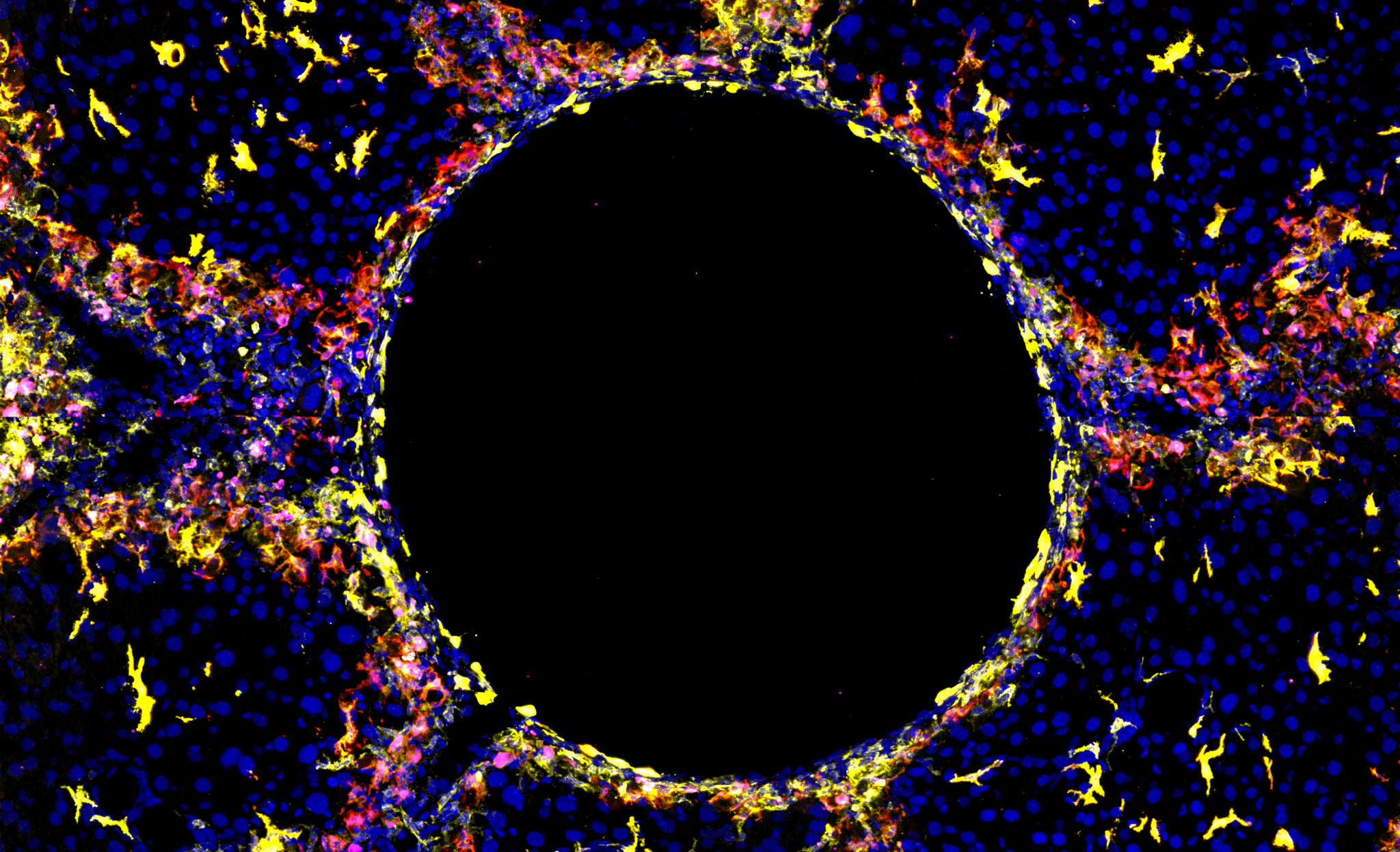
Specific types of liver immune cells are required to deal with injury
Liver immune cells are not static following an injury, as previously thought, but they adapt to the new microenvironment and participate in tissue repair
Ghent, 24 January 2025 – Our livers contain many different types of immune cells. New research by the team of Prof. Charlotte Scott (VIB-UGent Center for Inflammation Research) and colleagues now reveals that a specific activation state of one of these cell types is required for tissue repair following injury. This suggests these cells may be useful as new therapeutic targets for various liver conditions. The work appears in the journal Immunity.
Liver immune cells
Macrophages are specialized immune cells located in every tissue of the body, where they play crucial roles in maintaining tissue homeostasis, responding to injury, and facilitating tissue repair. In the healthy liver, most macrophages are classified as Kupffer cells (KCs). However, upon liver injury, as seen, for example, in obesity, another subset of macrophages called lipid-associated macrophages (LAMs) is recruited.
The specific functions of these cells in the context of liver injury were however unclear. Do KCs adapt upon injury to help heal the liver or is this a function for LAMs?
New work by the team of Prof. Charlotte Scott (VIB-UGent) and colleagues has addressed this specific question demonstrating that the LAM phenotype is critical for liver repair. Moreover, this research revealed that the KCs are not static post-injury, as previously thought and instead adapt to the new microenvironment also taking on a LAM-like phenotype, allowing them to also participate in the repair.

Heterogeneity and plasticity
“We used several advanced research methods, such as single-cell RNA sequencing and spatial transcriptomics, to investigate the heterogeneity, locations, and functions of hepatic macrophages in multiple injury settings,” says Dr. Ania Bujko, from the Scott lab and co-first author of the study, “this allowed us to identify similar populations of LAMs and LAM-like KCs irrespective of the injury type, which were found specifically at the site of injury. This finding was exciting as it suggests these cells may be useful therapeutic targets across various liver diseases.”
However, before the usefulness of these cells as a therapy could be considered, their functional contribution to injury and repair had to be assessed.
“To determine what these populations do in repair, we made use of different animal models to selectively delete genes of interest from the LAMs, LAM-like KCs, or both cells in the various liver injury contexts,” says Federico De Ponti, PhD student in the Scott lab and co-first author of the study. “This revealed that expression of the Trem2 gene is essential in at least one of these populations (LAMs or LAM-like KCs) for the efficient clearance of dying and injured liver cells”.

In other words, loss of TREM2 from both populations prevented repair, whereas repair could occur when expression was maintained in at least one of the populations. So, TREM2 in liver macrophages is critical for promoting tissue repair and preventing excessive fibrosis, suggesting its potential as a therapeutic target.
In addition, the study also demonstrates that the LAM identity is induced in the different macrophage populations through their uptake and clearance of dying cells at the site of injury.
“As this study highlights LAM-like macrophages as a potential therapeutic target to improve liver repair,” says Prof. Scott, “the information that clearance of dying cells drives this activation state could be utilized to generate such cells for therapeutic purposes, bringing us one step closer. While there are still a few questions remaining regarding these cells, that we are now investigating, this study demonstrates that the complex dynamics of hepatic macrophages and their functional roles in liver injury and repair could have significant implications for a variety of liver conditions.”
Publication
Spatially restricted and ontogenically distinct hepatic macrophages populations are required for tissue repair. De Ponti, Bujko, et al. Immunity, 2025.
Funding
ERC, FWO, Wellcome Trust, and German Research Foundation.
Gunnar De Winter