Tiny antennas on cells offer new ALS insights
Leuven, 20 December 2024- Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease that affects motor neurons. The average life span after diagnosis of this incurable disease is two to five years. In the relentless pursuit of understanding the cause of motor neuron death, scientists from KU Leuven and the VIB Center for Brain and Disease Research have identified an intriguing new lead: tiny, antenna-like structures 0n cells called primary cilia. Their study, published in Brain, could open a potential new avenue for therapeutic development.
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is the most common degenerative motor neuron disease in adults. It is characterized by a selective loss of motor neurons, resulting in progressive muscle weakness and paralysis, as well as swallowing and speech difficulties. Patients usually succumb to the disease within 2 to 5 years after symptom onset. Despite extensive research, the mechanisms of motor neuron death remain elusive, and there are currently no effective treatments to halt or reverse the progression of the disease. Now, researchers are pointing to the dysfunction of cilia, microscopic antennas of cells, essential for receiving and processing vital signals.
A broken antenna
In 2016, an international consortium led in Belgium by Prof. Philip Van Damme, neurologist at UZ Leuven and scientist at the KU Leuven Neuroscience department, identified C21orf2 as a new ALS-related gene. Mutations in C21orf2 were already known to disrupt cilia in other diseases. This prompted the team to investigate how these mechanisms play out in ALS.
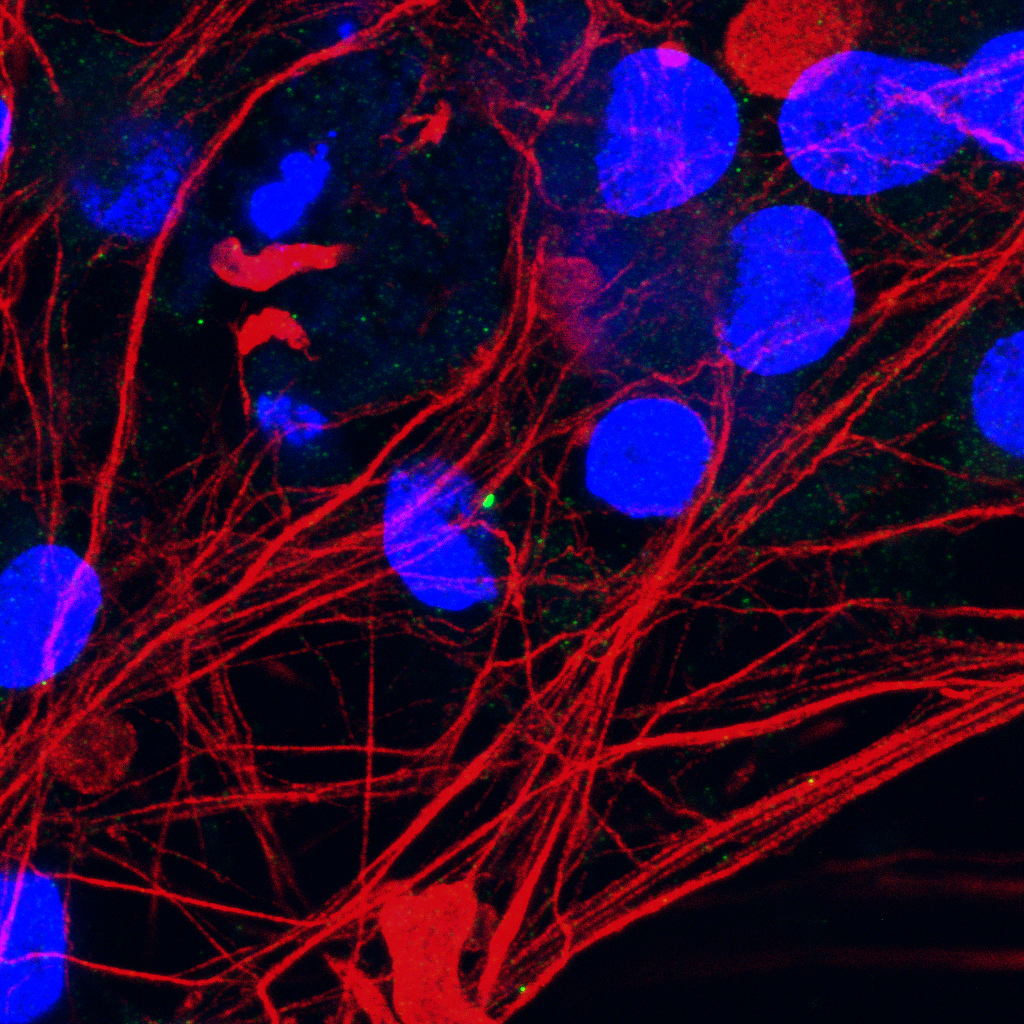
The study, in collaboration with researchers from the lab of Prof. Ludo Van Den Bosch at VIB-KU Leuven, revealed that mutations in C21orf2 impair the formation and structure of primary cilia. Motor neurons derived from patients with C21orf2 mutations had fewer cilia, and the cilia that remained were abnormally short.
“This structural damage prevents proper signal transmission,” says Dr. Mathias De Decker, first author. “We saw that the sonic hedgehog (Shh) pathway—a key pathway for motor neuron health was disrupted. When this happens, motor neurons struggle to form essential connections between nerves and muscles, known as neuromuscular junctions.”

Restoring the signal
Further experiments showed that restoring C21orf2 levels in mutated cells repaired the cilia defects, restored Shh signaling, and rescued neuromuscular junction formation. This discovery highlights primary cilia as a potential therapeutic target in ALS.
Strikingly, the researchers also observed similar cilia defects in motor neurons from ALS patients with mutations in one of the most common genetic causes of ALS, C9orf72. This suggests that cilia dysfunction might not be limited to one genetic subtype but could represent a broader problem in ALS biology.
Prof. Philip Van Damme: “These observations raise many questions and open avenues for further research. Overexpression of C21orf2 could rescue the cilia defects and formation of neuromuscular junctions, suggesting that targeting primary cilia dysfunction could become a therapeutic strategy for ALS.”
Questions from patients
A breakthrough in research is not the same as a breakthrough in medicine. The realizations of VIB researchers can form the basis of new therapies, but the development path still takes years. This can raise a lot of questions. That is why we ask you to please refer questions in your report or article to the email address that VIB makes available for this purpose: patienteninfo@vib.be. Everyone can submit questions concerning this and other medically-oriented research directly to VIB via this address.
Publication
C21ORF2 mutations point towards primary cilia dysfunction in amyotrophic lateral sclerosis. De Decker, et al. Brain, 2024. DOI: /10.1093/brain/awae331
Funding
The research (team) was supported by the Research Foundation Flanders (FWO), grants from KU Leuven, Opening the Future Fund (KU Leuven), the Agency for Innovation by Science and Technology, the ALS Liga België, the National Lottery of Belgium, the KU Leuven funds ‘Een Hart voor ALS,’ ‘Laeversfonds voor ALS Onderzoek,’ the ‘Valéry Perrier Race against ALS Fund’, the European E-Rare-3 project INTEGRALS, the European E-Rare-3 project MAXOMOD, Stichting ALS Nederland (TOTALS, ALS-ona-chip, GoALS), the E. von Behring Chair for Neuromuscular Disorders, and the European Research Council (ERC).
India Jane Wise
.png)


