When faster is not better: New study links premature development of human neurons to brain developmental disorders
Leuven, 7 August 2024—The mechanisms underlying intellectual disabilities or autism remain largely unknown. Researchers in the labs of Prof. Pierre Vanderhaeghen and Prof. Vincent Bonin at the VIB-KU Leuven Center for Brain & Disease Research and NERF have discovered that mutations in a gene called SYNGAP1 disrupt the prolonged development of human neurons, which is thought to be essential for normal cognitive function. Their work has interesting implications for our understanding and treatment development for intellectual disabilities or autism and appears in Neuron.
The human brain is notable among mammals for its remarkably prolonged development. Unlike in other animals, neurons in our brain, particularly in the cerebral cortex–the primary site of cognitive functions–take years to fully mature. This process, known as neoteny, is thought to be critical for developing some of the advanced cognitive functions characteristic of our species. Disruptions in this prolonged development could underlie some forms of intellectual disability and autism. Until now, this hypothesis had never been tested in human neurons.
A window into the maturing brain
Introducing the gene SYNGAP1. Previous studies found that mutations in this gene are a major cause of these conditions. However, the specific effects of its disruption on human cortical neurons remained largely unknown. Until recently, a major obstacle in studying human brain developmental diseases was the lack of reliable experimental methods to observe human cortical neuron development in a living brain.
Now, scientists at the VIB-KU Leuven Center for Brain & Disease Research and NERF (Neuro-Electronics Research Flanders, empowered by imec, KU Leuven, and VIB) have revealed that SYNGAP1 is crucial for the prolonged developmental timeline of human cortical neurons. This establishes a link between acceleration of neuronal development and intellectual disability and autism.
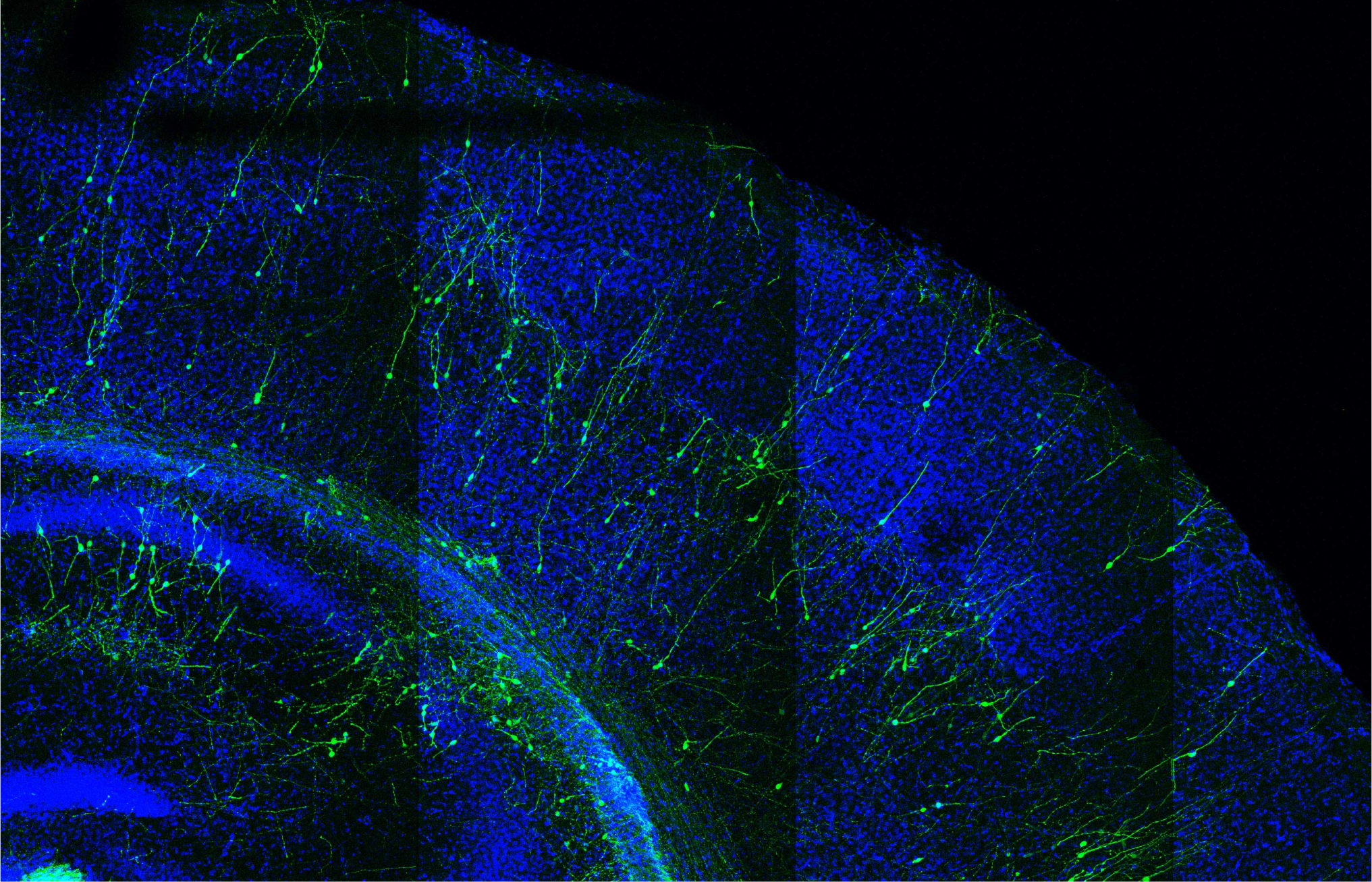
To investigate how the SYNGAP1 mutation affects human neuron development in vivo, the researchers used a xenotransplantation model: they grafted human neurons with the SYNGAP1 mutation into the brains of mice and subsequently studied their development and function.
Faster is not better
The researchers examined the effects of the mutation of transplanted human neurons in the mouse brain at the circuit level—connections between neurons that serve specific functions in the brain.
“We saw that the SYNGAP1 mutant neurons looked normal in most aspects, but that they displayed a strong acceleration of their development. Most strikingly, they connected much faster with other neurons,” explains Dr. Ben Vermaercke, first author of the paper.
In particular, Dr. Vermaercke and his colleagues discovered that the deficient neurons integrated faster into cortical circuits and responded to visual stimuli months ahead of the normal developmental schedule, indicating that the faster maturation of the neurons led to precocious functionality of the neurons within brain circuits.
Prof. Pierre Vanderhaeghen adds: “This accelerated development of SYNGAP1 mutant neurons could alter the early function and plasticity of infant brain circuits, although this needs to be studied further by experimental and clinical investigations. The important role of neoteny for normal human brain development highlights how its disruption can lead to neurodevelopmental diseases. Early defects in the development of human cortical neurons could have important implications for the diagnosis and treatments of the patients affected by SYNGAP1, and potentially in patients presenting other forms of intellectual disability or autism.”
Prof. Vincent Bonin concludes: “The transplantation model we developed enables, for the first time, the study of human neuronal diseases in vivo at both the functional and circuit levels. This breakthrough constitutes a promising model for understanding neurological diseases and testing new treatments.”
Publication
SYNGAP1 deficiency disrupts synaptic neoteny in xenotransplanted human cortical neurons in vivo. Vermaercke, Iwata, et al. Neuron, 2024. DOI: 10.1016/j.neuron.2024.07.007
Funding
The research (team) was supported by the Research Foundation Flanders (FWO), Grants of the European Research Council (NEUROTEMPO), the Foundation GENERET, the C1 KU Leuven Internal Funds Programme, the EOS Programme, ERANET EPINEURODEVO, NSC-Reconstruct and the Belgian Queen Elizabeth Foundation.
India Jane Wise
Joran Lauwers
About the VIB-KU Leuven Center for Brain & Disease Research
Scientists at the VIB-KU Leuven Center for Brain & Disease study how brain cells are organized and how they communicate with each other. These mechanisms reveal and provide insights into what goes wrong in brain diseases such as Alzheimer's, Parkinson's, ALS, and dystonia. This basic work should ultimately lead to new drugs for use against these currently incurable diseases.
About NERF
Neuro-Electronics Research Flanders (NERF) is a not-for-profit academic research initiative empowered by imec, KU Leuven and VIB, with the ultimate goal of forming a thorough understanding of brain function at multiple levels of detail ranging from cells and circuits to behavior. New insights into the operation of brain circuits are empowered by the development of novel technologies that integrate neurobiology and nano-scale engineering. NERF develops novel electronic, chemical and optical tools to monitor and manipulate brain circuits with high spatial and temporal resolution. More info: www.nerf.be.
%20transplanted%20with%20human%20neurons%20(in%20green).png)





