Decoding the satiety signal
Research reveals how leptin and its receptor work together to regulate hunger signals
The protein hormone leptin signals our brains that we are full and can stop eating. To send that signal, leptin has to bind to a receptor on the cells that need to receive the message. Researchers at the VIB-UGent Center for Inflammation Research, collaborating with colleagues at Osnabruck University (Germany) and the Belgian biotechnology company PUXANO, have now revealed how leptin and its receptor work together. Their arresting research results appear in the leading scientific journal Nature Structural & Molecular Biology and may spark new approaches in treating obesity, a major metabolic condition worldwide.
The satiety hormone
In 1994, the American scientist Jeffrey Friedman discovered the hormone leptin. He wanted to understand why some lab mice strains could not stop eating. Eventually, he zeroed in on leptin, now also known as the satiety hormone, which regulates body weight and other biological processes involved in immunity, fertility, and cancer, for example.
Leptin is made by white fat cells and the gastric mucosa. When the leptin levels are high, our brains receive the signal that our energy stores are sufficient and we don't need to eat. We feel full, in other words. That 'I'm full' signal is passed along by leptin when it binds to the leptin receptor (LEP-R) on the surface of brain cells.
Dr. Kenneth Verstraete, who led the research with prof. Savvas Savvides (VIB-UGent Center for Inflammation research): "The receptor for Leptin has six different forms with varying activity levels. The longest form of the receptor can send signals to the brain and is linked to energy balance and food intake. The leptin-receptor assembly took us completely by surprise! It is constructed by three leptin molecules connecting three receptor molecules, which offers important new insights to understand how leptin functions."
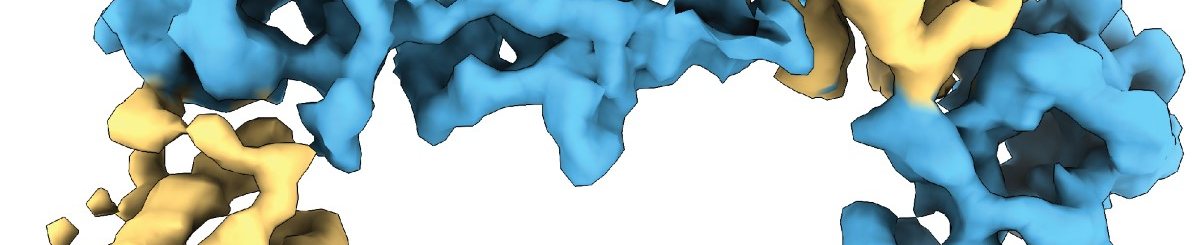
Visualizing the leptin-LEP-R receptor complex
The researchers used X-ray crystallography and Cryo-Electron Microscopy to determine the 3D-structure of the leptin- LEP-R assemblies to near-atomic resolution and single-molecule tracking to confirm such complexes on living cells. Such an innovative combination of methods was critical to the project's success. The researchers found that leptin induces a unique type of receptor assembly and undergoes structural changes that activate its binding to LEP-R.

Dr. Alexandra Tsirigotaki, first author of the study: "We know that specific mutations in leptin and the leptin receptor are associated with severe obesity. Our findings now explain such clinically important mutations and will be an important resource for the large community studying leptin signaling in basic and clinical science."
This new understanding of how leptin interacts with its receptors at the cell surface has important implications for understanding how the hormone works in the body.
Prof. Savvas Savvides: "Collectively, our work will help to consolidate two decades of research on the role of leptin in physiology and disease and will catalyze the development of treatments for leptin dysfunction and the creation of new variants of leptin with improved properties."
Publication
Mechanism of receptor assembly via the pleiotropic adipokine Leptin. Tsirigotaki et al. Nature Structural & Molecular Biology, 2023.
Gunnar De Winter